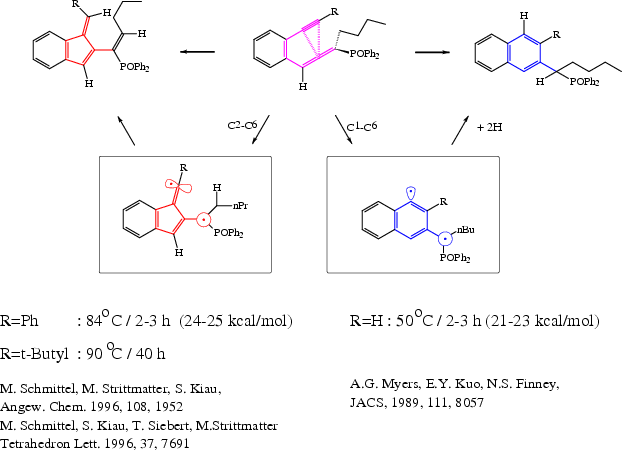
In 1989 Myers et. al. [1] published a
C-1 C-6 6-ring cyclization of enyne-ketenimines. Seven years later
Schmittel et. al.
[2,3]
discovered a C-2 C-6 5-ring cyclization. The two reactions are schown below:

After the experimental discovery quantum chemical screening calculation on
a model system were successfully carried
out [4,5,6,7] to predict the gain of synthesis with varying rests R.
The theoretical results are schown below:
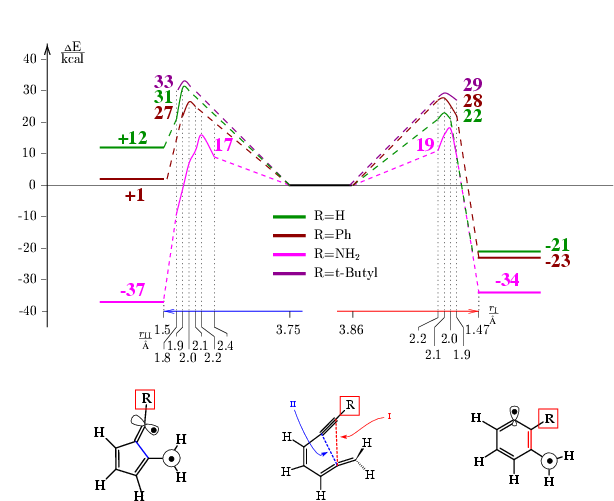
From the theoretical point of view the description of the transition states
requires a multi-reference wavefunction due to the existence of the diradical
structure of the molecule.